Research Article
The incidence of hemodynamic and respiratory adverse events in morbidly obese presenting for Bariatric surgery

Veronica Atterhem, Magnus Hultin and Tomi Myrberg*
Department of Surgical and Perioperative Sciences, Anesthesiology and Critical Care Medicine, Sunderby Research Unit, Umeå University, Umeå, Sweden*Address for Correspondence: Tomi Myrberg, Department of Surgical and Perioperative Science, Anesthesiology and Critical Care Medicine, Umeå University, 901 87 Umeå, Sweden, Tel: +46 920 282 000; Email: tomi.myrberg@umu.se
Dates: Submitted: 06 July 2018; Approved: 24 July 2018; Published: 25 July 2018
How to cite this article: Atterhem V, Hultin M, Myrberg T. The incidence of hemodynamic and respiratory adverse events in morbidly obese presenting for Bariatric surgery. Int J Clin Anesth Res. 2018; 2: 009-017. DOI: 10.29328/journal.ijcar.1001006
Copyright License: © 2018 Atterhem V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Morbid obesity; Bariatric surgery; Desaturation; Hypoxemia; Anaesthesia; Venous return; Anesthesia methods
Abbreviation: BMI: Body Mass Index; CPAP: Continuous Positive Pressure Ventilation; ERAS: Enhanced Recovery of Abdominal Surgery; MO: Morbidly Obese Patients; TBW: Total Body Weight; LBW: Lean Body Weight; OSAS: Obstructive Sleep Apnoea Syndrome; OHS: Obesity Hypoventilation Syndrome; PEEP: Positive End-Expiratory Pressure; RWL: Rapid-Weight-Loss Diet; SAP: Systolic Blood Pressure; Spo2: Peripheral Saturation; RSI: Rapid Sequence Induction of Anesthesia
Abstract
Context: Perioperative management of morbidly obese patients undergoing bariatric surgery is challenging. Lacking standardized perioperative protocols, complication rates may be high. This retrospective study aims to quantify the incidence of significant blood pressure decreases on induction of anesthesia and intraoperative hypoxemia, before implementation of a standardized protocol designed for bariatric surgery.
Design: Retrospective, observational study.
Setting: A 250-bed county hospital in northern Sweden.
Subjects: 219 morbidly obese patients (body mass index > 35 kg/m2) who underwent bariatric surgery between 2003 and 2008.
Main outcome measures: Incidence of systolic blood pressure (SAP) falls to less than 70% of the preoperative baseline during induction of anesthesia and incidence of perioperative hypoxemia.
Results: The incidence of confirmed SAP falls to below 70% of baseline at induction of anesthesia was 56.2% (n = 123/219). This incidence rose with increasing age (p < 0.001) but not with body mass index (BMI). 3.7% (n = 8/219) of cases were marked as difficult intubations. A transient period of hypoxemia was observed in 6.8% (n = 15/219) and was more common with increasing BMI (p = 0.005). Fourteen different drug combinations were used in the study population. Of those administered an induction anesthetic drug, 72.6% (n = 159/193) were given an overdose when calculated by lean body weight, but this did not correlate significantly to SAP falls (p = 0.468).
Conclusion: The incidence of a significant blood pressure fall upon induction of anesthesia was common. The incidence of airway and ventilation problems were low. Overdosing of anesthetics and excessive variation in applied anesthesia methods were found.
Background
Morbid obesity (MO) is a significant risk factor for cardiovascular disease, cancer, diabetes, sleep apnoea, and increased overall mortality [1,2]. Bariatric surgery may, in addition to reducing weight, reduce co-morbidities [3], but MO presents several challenges to an anesthesiologist and requires specialized perioperative management. These patients have an altered body habitus, often with massive fat deposits on face and abdomen, chronic increased intra-abdominal and intra-cranial pressures, and cardiopulmonary involvement.
Anesthesia may compound these obesity-related problems. Lung volumes are frequently reduced due to the increased mass around the thorax and predisposes even a conscious, supine MO patient to hypoxemia [4-6]. Preoxygenation is crucial since MO have a reduced safe apnoea time [7] and mask-bag ventilation is frequently a challenge [8], and the incidence of intubation problems in MO has been reported as high as 13% [4]. The functional residual lung capacity of a MO patient may at induction of anesthesia decrease by 50% compared to a non-obese patient’s reduction of 20% [8]. Additionally, the pneumoperitoneum crucial for laparoscopic surgery adds approximately 15 mmHg to intra-abdominal pressure which may further decrease lung volumes and respiratory compliance, and may through hindrance of venous return reduce cardiac output, especially in hypovolemic patients [4,5,9]. Atelectasis development is often profound in MO and resolves more slowly compared to the non-obese population, so the increased risk of hypoxemia in MO lingers in the post-operative 24 hours [10].
Intraoperative hypotension is an adverse hemodynamic event known to increase both postoperative hospital stay [11] and postoperative morbidity [12]. Long-term severe obesity may cause a hypervolemic circulation with venous hypertension, concomitant with hypertrophy, diastolic dysfunction and biventricular dilatation, i.e. obesity cardiomyopathy [4,13,14]. Clinically these problems are manifested by MO’s cardiac index falling 17-33% upon induction of anesthesia compared to non-obese patients’ decreases of 4-11%, raising MO’s risk for significant hypotension [4,6]. In addition to appropriate dosing of anesthetics [15,16], the cornerstone of cardiovascular stability is perioperative euvolemia [5,17]. However, to facilitate laparoscopic bariatric surgery and decrease the risk for surgical complications [18], patients must undergo a pre-surgical rapid weight loss regimen, which has been shown to leave 71.4% in a hypovolemic state [9], further exacerbating the risk for significant intraoperative hypotension, especially under pneumoperitoneum [5].
The aim of this study was to quantify the incidence of intraoperative hypoxemia and significant blood pressure falls before the implementation of a standardized protocol for bariatric surgery.
Methods
The anesthesia records of 231 morbidly obese patients who underwent bariatric surgery between the 2003 and 2008 were retrospectively examined. Of interest were baseline systolic blood pressure, blood pressure readings once every five minutes, saturation values, exact anesthetics used and dosages, and eventual notations on intubation difficulties. Data on height, weight, pre-surgery weight loss, co-morbidities, and medications were obtained from patient journals. The main query concerned the incidence of systolic arterial pressure (SAP) falls below 70% of baseline upon induction of anesthesia, where the “fall value” was the lowest registered SAP in the first 10 minutes post-induction. Secondary queries were incidence of relative overdosing of anesthetics, difficult intubations, intraoperative hypoxemia (defined as peripheral saturation (SpO2) ≤ 92%), and SAP falls below 70% of baseline later than the 10 minutes post-induction period (“later-lowest” SAP).
Characteristics of the study population
The study group consisted of 231 patients who underwent open or laparoscopic gastric bypass or “other volume reductive procedure” at Sunderby Hospital in 2003 - 2008. Twelve patients were excluded from the data pool due to BMI < 35, leaving 219 participants. 72% of the patients were female, mean age was 40.8 years (range 19 - 65). 31% were younger than 35 years, 48% were 36 - 50 years, and the remainder > 51. The mean BMI was 44.2 kg/m2 (range 35.1 - 69.1). Of these, 27% had a BMI ≤ 40, 56% between 40 and 50, and 17% had BMI > 50. All patients were subjected to a liquid diet for three weeks prior to surgery, and the mean preoperative weight loss was 8.5 ± 3.6 kg.
Co-morbidities and use of anti-depressive medication were common in the study population. Clinic praxis is to pause anti-hypertensive drugs at the day of surgery. Patient characteristics such as gender, age, BMI, and medication are summarized in Table 1.
| Table 1: Characteristics, co-morbidities, and regular medication n = 219 | |
| Characteristics | |
| Age (years) | 40.8 (range 19 – 65) |
| Gender, Female (%) | 72 |
| BMI (kg/m²) at the day of surgery | 44.2 (range 35.1 – 69.1) |
| Smoker (current and/or previous) (%) | 40 |
| Co-morbidities | |
| Hypertension (%) | 40 |
| Diabetes mellitus (%) | 24 |
| Bronchial asthma (%) | 25 |
| COPD (%) | 1 |
| OSAS/OHS (%) | 9 |
| Angina pectoris (%) | 6 |
| Renal failure (%) | 1 |
| Medication | |
| Beta-blockers (%) | 19 |
| ACE/ARB (%) | 25 |
| Calcium channel blockers (%) | 5 |
| Nitroglycerine (%) | 4 |
| Antidepressants (%) | 20 |
Baseline systolic blood pressure and Sp02 values
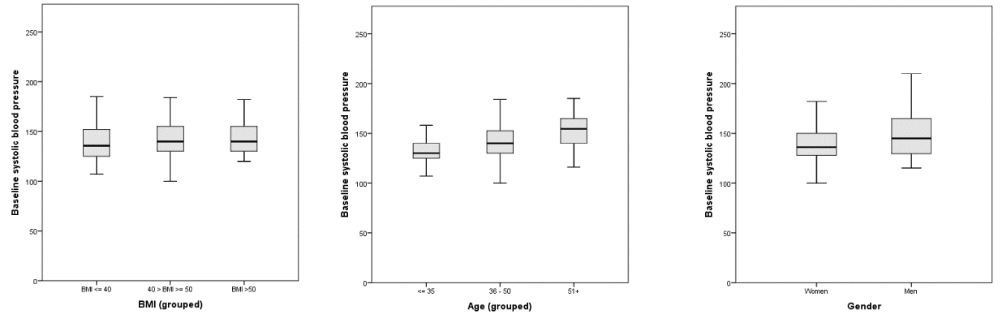
An automatic non-invasive blood pressure (NIBP) measurement and peripheral oxygen saturation (SpO2) were registered prior to preoxygenation in a supine position. Each operating theatre had access to an appropriate-sized NIBP-cuff for MO individuals. In seven cases there was no recorded baseline value for the systolic blood pressure, thus 212 cases was used for calculation of the baseline (pre-anaesthesia) SAP. The mean baseline SAP was 142 mmHg (range 100-210). The baseline systolic blood pressure values are summarized and presented by BMI, age and gender in Figure 1.
Anesthesia procedures
An anticholinergic agent (atropine, 0.25-1 mg) was administered intravenously before preoxygenation period in most patients (n = 195/219). Since there were no records of whether or how any preoxygenation manoeuvres were performed in the study population, the quality of preoxygenation before induction could not be evaluated. The assumption was made that preoxygenation was done according to clinic standard praxis of fresh oxygen gas flow (FiO2 0.9) on a mask for several minutes with a low PEEP around 5 cm H2O.
Patients were mostly given an induction dose of propofol or thiopenthone, often together with suxamethonium and fentanyl, and thereafter anesthesia was maintained with combinations of sevoflurane gas, remifentanyl infusions, and/or propofol infusions. Note that while sevoflurane gas was administered only after induction and intubation, whereas anesthesia was maintained by infusions started just prior to administration of the induction dose, which means that the total drug load in the patient’s system varied at induction depending upon the exact anesthesia protocol. Some patients may also have been administered triazolam (0.25 mg p.o.) if anxious prior to surgery. Administered anesthetic doses were evaluated by total body weight (TBW, on the day of surgery) and lean body weight (LBW) estimates. LBW was calculated by height (in cm) – 100 for men and height (in cm) – 105 for women.
Statistics
Data were analyzed and processed in the Statistical Package for Social Sciences (SPSS) version 24.0 and examined by age, BMI, gender, co-morbidities and medication. The two-tailed Student’s test was used for BMI and age. Where the sample size was small, the non-parametric Mann-Whitney test was used. When appropriate, Pearson Chi-Square test was performed for binominal data, and an ANOVA when binomial data were compared to normally distributed data. Two-tailed p-values ≤ 0.05 were considered statistically significant.
Results
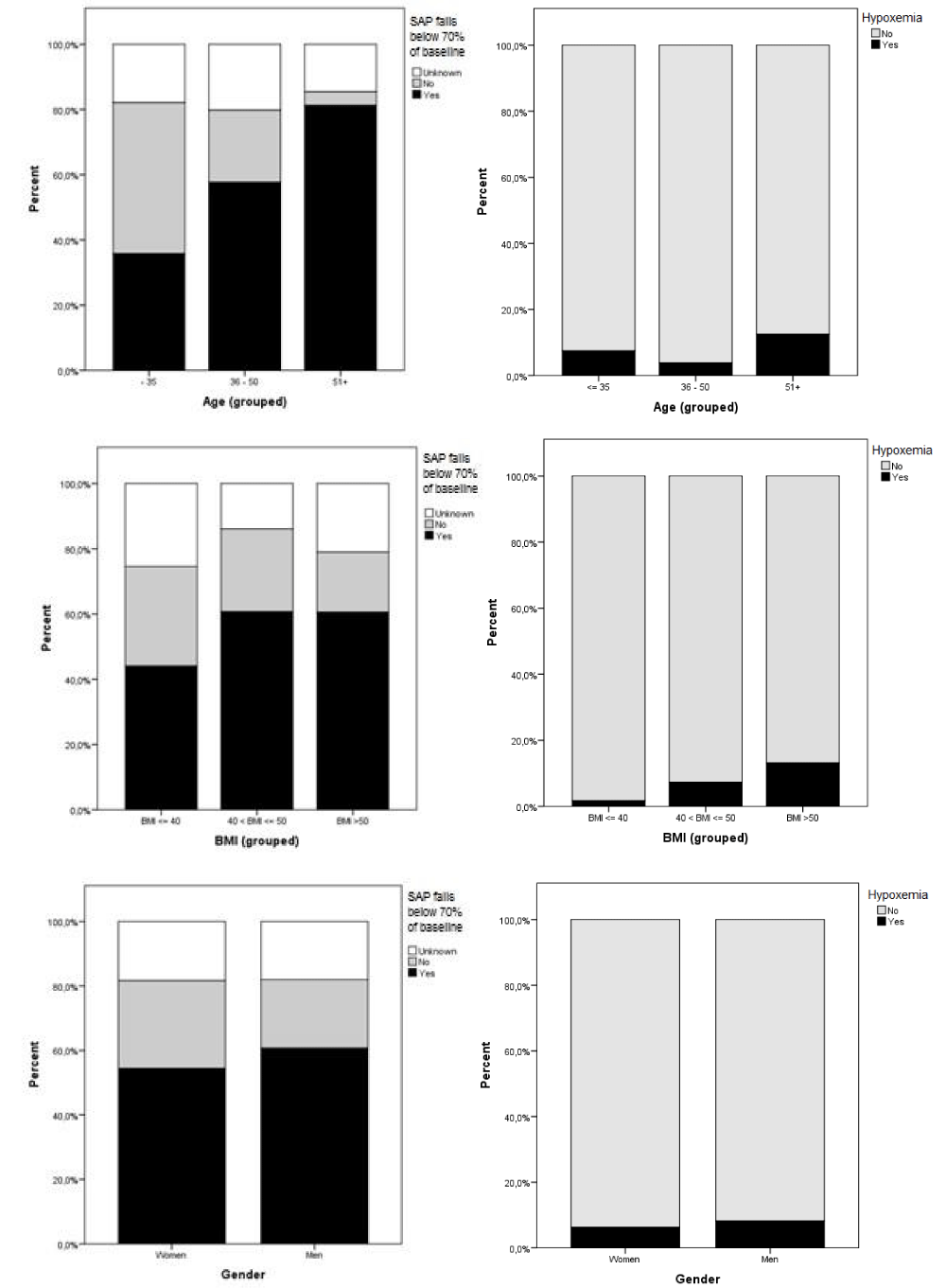
The incidence of confirmed SAP falls below 70% of baseline at anesthesia induction was 56.2% (n = 123/219). The mean post-induction SAP was 91 mmHg (range 47-150), calculated from 182 cases (in 37 cases no SAP values were registered within ten minutes of induction). In 18.3% (n = 40/219) the question of whether they fell below 70% of baseline SAP was left unanswered due to missing baseline (n = 7/219) and post-induction (n = 37/219) SAP values; two cases were missing both, and for two cases lacking their baseline SAP a “yes” to SAP falls has been assumed since the post-induction value was below 70 mmHg. A significant increase in the incidence of SAP falls was found with increasing age (p < 0.001) but not with increasing BMI. Patients with hypertension also showed a greater risk for SAP falls (p < 0.001). Intraoperatively, the mean “later-lowest SAP” was 80 mmHg (range 52 - 110), with 84.5% (n = 185/219) of patients dropping below 70% of pre-induction baseline during anesthesia. At some point during their procedures, a transient period of hypoxemia was observed in 6.8% (n = 15/219) patients, an incidence which rose with increasing BMI (p = 0.005, 95% CI -7.8 to – 1.4), but not with age. 3.7% (n = 8/219) were noted as having difficult intubations, but no significant correlation could be found between intubation difficulties and BMI or age. In addition, no significant difference in incidence was found between genders for SAP falls, hypoxemias or intubation difficulties. The incidence of SAP falls below 70% of baseline and the incidence of hypoxemia presented by age, BMI, and gender are summarized in Figure 2.
Figure 2: The incidence of SAP falls below 70% of baseline, and the incidence of hypoxemia, presented by age, BMI, and gender. Percentages shown are of all 219 cases, thus the unknown results for the main query are included.
Anesthesia
Patients were administered either a propofol (n = 106/219) or a thiopenthone (n = 84/219) induction, or a combination of the two (n = 3/219). In some anesthesia records the induction drug and/or dose was illegible (n = 7/219) or there was no induction drug noted (n = 19/219). Without clear correlation to any anesthesia protocol, some patients at induction also received suxamethonium (n = 179/219), alfentanil (n = 38/219), and/or fentanyl (n = 101/219). For maintenance, without clear correlation to choice of induction drug, patients were given either sevoflurane gas (n = 77/219) or an infusion of remifentanil with sevoflurane gas (n = 118/219), or infusions of remifentanil and propofol (n = 20/219), or infusions of remifentanil and propofol in combination with sevoflurane gas (n = 4/219). Absolute mean administered dose of propofol was 230 ± 72 mg, of thiopenthone 439 ± 108 mg, fentanyl 161 ± 59 µg (total dose administered within ten minutes of induction), and suxamethonium 107 ± 25 mg. Dose per total body weight (TBW) of propofol was 1.85 ± 0.58 mg/kg, of thiopenthone 3.52 ± 0.79 mg/kg, fentanyl 1.28 ± 0.50 µg/kg, and suxamethonium 0.83 ± 0.23 mg/kg. Dose per estimated lean body weight (LBW) of propofol was 3.52 ± 1.03 mg/kg, thiopenthone 6.94 ± 1.76 mg/kg, and fentanyl 2.51 ± 0.93 µg/kg. More propofol and thiopenthone (mg/kg LBW) was given to women (3.58 ± 0.96 and 7.20 ± 1.78) compared to men (3.36 ± 1.22 and 6.22 ± 1.51), but the difference was only significant for thiopenthone (p = 0.023), not for propofol (p = 0.328). The above dose calculations for thiopenthone and propofol do not include the three cases where both were used together.
For propofol, doses greater than 2.5 mg/kg LBW were considered overdoses, as were thiopenthone doses greater than 6 mg/kg LBW. Of 193 patients given an induction dose of either or both, 72.6% (n = 159/193) were given an overdose. Significantly more women than men received an overdose (p = 0.043), but there was no correlation between overdoses and SAP falls (p = 0.317), not even when split between propofol (p = 0.615) and thiopenthone (p = 0.187). There was no significant correlation between choice of induction method and SAP falls (p = 0.468). Perioperative and 30-day mortality was zero.
Discussion
The main objective of this retrospective study was to quantify the incidence of significant blood pressure falls upon induction of anesthesia, and the incidence of intraoperative hypoxemia, for the period before our clinic implemented of a standardized protocol for bariatric surgery [17,19]. A significant blood pressure fall was defined as a drop in systolic arterial pressure (SAP) to below 70% of baseline. There is no international consensus on the definition of hypotension: one study lists over 140 definitions and notes that hypotension incidence varies depending upon the definition [20]. SAP falls below 70% of baseline is the definition of hypotension when administering spinal anesthesia [21]. At our clinic, this level is the currently acceptable intraoperative blood pressure drop [17], consistent with other in-house research.
This study found significant post-induction SAP falls in 56.2% (n = 123/219) of bariatric surgery patients, and demonstrated that incidence increased with patient age and with hypertension. That SAP falls are more related to increasing age than to increasing BMI has been previously shown. Other known independent predictors of clinically significant intraoperative hypotension are pre-existing hypertension, anti-hypertensive drugs, age, arterial stiffness and the use of propofol [12,22]; it has been shown that in the MO population even young (< 40 years) individuals suffer these cardiorespiratory co-morbidities [23,24].
This retrospective study of the anesthetic management of bariatric surgery patients before standardization has shown not only a high incidence of adverse events, especially SAP falls, but also how the choice and dosage of anesthetics may vary without standardization. When recalculated by lean body weight (LBW), 72.6% of propofol and thiopenthone induction doses were found to have been overdoses. These doses were possibly calculated based on total body weight (TBW) instead of LBW. We found no clear connection between these overdoses and the high rate of SAP falls, likely due to the diverse field of simultaneously administered drugs and the patients’ co-morbidities (no connection was found when the anesthesia protocols were analyzed separately). No further attempt has been made to draw any conclusions from these sub-groups, but it is clear that the management of these patients was highly inconsistent, and that the induction doses were frequently too high. It is easy to speculate that this added to adverse events such as SAP falls at induction.
As opposed to the many preceding reports on either post-inductive or post-operative hypoxemia, this study registered transient hypoxemias throughout the procedure. Their incidence was 6.8%, which was found to increase with higher BMIs. That the propensity for hypoxemia grows with increasing BMI has been previously reported [25], as has the correlation between atelectasis formation and body weight [26], as well as that the alveolar-arterial oxygen tension gradient during anesthesia rises linearly with BMI [27]. To reduce the risk of hypoxemia, one may employ intra-operative alveolar recruitment manoeuvres followed by positive end-expiratory pressure [10,26].
The incidence of intubation problems in this study was 3.7%. Other studies report both higher and lower incidences of intubation problems related to MO [28], while some claim MO has no predictive value for intubation difficulties at all [29]. One study compared the morbidly obese (BMI 40-49.9) with the super-obese (BMI 50-59.9) and the super-super-obese (≥ 60) and concluded that super-obesity was not a predictor of difficult intubation [30].
Our clinic now has fewer adverse events, and since October 2007 all bariatric operations have been registered in the Scandinavian Obesity Surgery Registry (SOReg) (http://www.ucr.uu.se/soreg/). In 2009, a PhD project at Umeå University was initiated to standardize the perioperative management of bariatric surgery patients. Throughout that project, no hemodynamic nor respiratory adverse events were recorded [17,19], and our current praxis is based on the method developed during that project.
Our current bariatric anesthesia procedure aims to optimize venous preload with colloid fluids [14,17], preoxygenate with CPAP and vital capacity breaths in the ramp position (created by Tempur-MED Intubation Pillow™) [31], and intubate using video-assisted laryngoscopes, which has been shown to reduce the risk of serious hypoxemias during tracheal intubation in MO [32]. At induction, propofol and alfentanil are dosed according to LBW, suxamethonium by TBW (maximal dose 150 mg), and rapid sequence induction (RSI) and RSI-bolus (high speed injection) are used consistently [15]. Short-acting non-depolarizing muscle agents (rocuronium and mivacurone) are dosed to maintain muscle relaxation. Remifentanil infusions are used intraoperatively, combined with sevoflurane with a low set MAC goal at 0.7-0.8. To optimize oxygenation, intraoperative recruitment manoeuvres, LBW-based tidal volumes, and PEEP 8 - 10 cm H20 are performed. To minimize the risk of postoperative nausea and vomiting, prophylactic antiemetics are given more generously than indicated by standard APFEL scores [33], and non-steroidal anti-inflammatory drugs [34] and occasionally clonidine are used to minimize the need of postoperative opiates. Standard blood pressure monitoring is generally non-invasive, but when preoperatively deemed appropriate, invasive blood pressure, cardiac output measurements and inotropic drugs are used [17]. Extubation is done in the ramp position. Postoperative CPAP is used for patients who have an OSAS and/or OHS diagnosis, with their own nasal CPAP-apparatus. However, to create a real ERAS praxis for bariatric surgery, our cooperation with pre- and post-operative ward units needs further refinement [35].
The results of this comparison between our previous modi operandi and our current methods of handling these patients leads us to conclude that perioperative management of the morbidly obese should be standardized in all anesthesia praxis. Regardless of surgical procedure, the MO patient is in need of thorough preoperative risk assessment and optimization, including euvolemia and positioning, to attain perioperative respiratory and cardiovascular stability [5,17,27,28]. This conclusion is supported by previous recommendations that the MO population be considered high-risk for cardiovascular and respiratory adverse events during anesthesia [24].
This study is limited first and foremost by how the retrospective data taken from anesthesia records suffers from missing values due to incomplete or illegible records. Moreover, the source material supplied no information on respiratory management, levels of venous return, or postoperative complications. Levels of preoperative venous return or intra-abdominal pressures in the study group were never registered and cannot now be assessed. The aim of the study was to quantify cardiorespiratory adverse events upon the induction of anesthesia and intraoperatively, and therefore incidences of postoperative complications (e.g. reoperation frequency, acute renal failure) were not studied.
Conclusion
In this retrospective study the incidence of a significant blood pressure fall upon induction of anesthesia was common. The incidence of airway and ventilation problems were low. Overdosing of anesthetics and excessive variation in applied anesthesia methods were found.
Declarations
Ethical Approval and consent to participate
Ethical approval (DNR 09-042M) for the study was granted by the regional ethics committee in Umeå, Sweden; Chairman Anders Iacobaeus; Samverkanshuset, Universitetsområdet, 90187 Umeå, Sweden on 30 October 2009. Due to the study design, formal consent was neither required nor obtained. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, the study design is retrospective. For this type of study, formal consent is not required. No identifying information is available in this article.
Availability of data and materials: The SPSS data files regarding of this manuscript are available from the corresponding author on reasonable request.
Authors’ Contributions: Substantial contributions to the data collection, analysis and article preparation was made by the author V.A. The study concept, design, analysis of the results and article preparation was made by the author T.M. M.H. contributed to analysis, interpretation of data and preparation of the article.
Acknowledgement
This research was supported by the Norrbotten County Council.
References
- Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013; 273: 219-234. Ref.: https://tinyurl.com/yctwa5xr
- Marshall NS, Delling L, Grunstein RR, Peltonen M, Sjöström CD, et al. Self-reported sleep apnoea and mortality in patients from the Swedish Obese Subjects study. Eur Respir J. 2011; 38: 1349-1354. Ref.: https://tinyurl.com/ycfl8rck
- Sjoholm K, Anveden A, Peltonen M, Jacobson P, Romeo S, et al. Evaluation of current eligibility criteria for bariatric surgery: diabetes prevention and risk factor changes in the Swedish obese subjects (SOS) study. Diabetes Care. 2013; 36: 1335-1340. Ref.: https://tinyurl.com/ybk8msyp
- Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000; 85: 91-108. Ref.: https://tinyurl.com/y9o54rth
- Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005; 241: 219-226. Ref.: https://tinyurl.com/y7rlb5hr
- Brodsky JB. Positioning the morbidly obese patient for anesthesia. Obes Surg. 2002; 12: 751-758. Ref.: https://tinyurl.com/yca5m66m
- Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997; 87: 979-982. Ref.: https://tinyurl.com/ybm38zab
- Murphy C, Wong DT. Airway management and oxygenation in obese patients. Can J Anaesth. 2013; 60: 929-945. Ref.: https://tinyurl.com/y8ap5rmz
- Poso T, Kesek D, Aroch R, Winso O. Rapid weight loss is associated with preoperative hypovolemia in morbidly obese patients. Obes Surg. 2013; 23: 306-313. Ref.: https://tinyurl.com/yczubse2
- Talab HF, Zabani IA, Abdelrahman HS, Bukhari WL, Mamoun I, et al. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth Analg. 2009; 109: 1511-1516. Ref.: https://tinyurl.com/ycjrdub3
- Reich DL, Hossain S, Krol M, Baez B, Patel P, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005; 101: 622-628. Ref.: https://tinyurl.com/y9orsbsk
- Alecu C, Cuignet-Royer E, Mertes PM, Salvi P, Vespignani H, et al. Pre-existing arterial stiffness can predict hypotension during induction of anaesthesia in the elderly. Br J Anaesth. 2010; 105: 583-588. Ref.: https://tinyurl.com/y9l8cfwz
- Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014; 56: 391-400. Ref.: https://tinyurl.com/ycer82c8
- Poso T, Kesek D, Aroch R, Winso O. Morbid obesity and optimization of preoperative fluid therapy. Obes Surg. 2013; 23: 1799-1805. Ref.: https://tinyurl.com/ybcnvlvz
- Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010; 105: i16-23. Ref.: https://tinyurl.com/y9pgh7ds
- Lemmens HJ. Perioperative pharmacology in morbid obesity. Curr Opin Anaesthesiol. 2010; 23: 485-491. Ref.: https://tinyurl.com/y8kd6sfp
- Poso T, Winso O, Aroch R, Kesek D. Perioperative Fluid Guidance with Transthoracic Echocardiography and Pulse-Contour Device in Morbidly Obese Patients. Obes Surg. 2014; 24: 2117-2125. Ref.: https://tinyurl.com/y9czf5h7
- Anderin C, Gustafsson UO, Heijbel N, Thorell A. Weight Loss Before Bariatric Surgery and Postoperative Complications: Data From the Scandinavian Obesity Registry (SOReg). Ann Surg. 2015; 261: 909-913. Ref.: https://tinyurl.com/ychqk4oc
- Poso T, Kesek D, Winso O, Andersson S. Volatile rapid sequence induction in morbidly obese patients. Eur J Anaesthesiol. 2011; 28: 781-787. Ref.: https://tinyurl.com/yc2e2spw
- Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, et al. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007; 107: 213-220. Ref.: https://tinyurl.com/y8wttpj5
- Miller RDE. Miller's Anaesthesia. 8th ed. Philadelphia: Churchill Livingstone. 2014; 1713.
- Gragasin FS, Bourque SL, Davidge ST. Vascular aging and hemodynamic stability in the intraoperative period. Front Physiol. 2012; 3: 74. Ref.: https://tinyurl.com/y6udfpk6
- Acree LS, Montgomery PS, Gardner AW. The influence of obesity on arterial compliance in adult men and women. Vasc Med. 2007; 12: 183-188. Ref.: https://tinyurl.com/ydx3y9k5
- Katkhouda N, Mason RJ, Wu B, Takla FS, Keenan RM, et al. Evaluation and treatment of patients with cardiac disease undergoing bariatric surgery. Surg Obes Relat Dis. 2012; 8: 634-640. Ref.: https://tinyurl.com/yd3gaubx
- Wani S, Azar R, Hovis CE, Hovis RM, Cote GA, et al. Obesity as a risk factor for sedation-related complications during propofol-mediated sedation for advanced endoscopic procedures. Gastrointest Endosc. 2011; 74: 1238-1247. Ref.: https://tinyurl.com/ydefrwxe
- Reinius H, Jonsson L, Gustafsson S, Sundbom M, Duvernoy O, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology. 2009; 111: 979-987. Ref.: https://tinyurl.com/ybdd5pa6
- O'Neill T, Allam J. Anaesthetic considerations and management of the obese patient presenting for bariatric surgery. Current Anaesthesia Critical Care. 2010; 21: 16-23. Ref.: https://tinyurl.com/ybsm5aof
- Gonzalez H, Minville V, Delanoue K, Mazerolles M, Concina D, et al. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth Analg. 2008; 106: 1132-1136. Ref.: https://tinyurl.com/yd6vnrtz
- Brodsky JB, Lemmens HJ, Brock-Utne JG, Vierra M, Saidman LJ. Morbid obesity and tracheal intubation. Anesth Analg. 2002; 94: 732-736. Ref.: https://tinyurl.com/ych7g5fb
- Leykin Y, Pellis T, Del Mestro E, Marzano B, Fanti G, et al. Anesthetic management of morbidly obese and super-morbidly obese patients undergoing bariatric operations: hospital course and outcomes. Obes Surg. 2006; 16: 1563-1569. Ref.: https://tinyurl.com/ycj48qug
- Dixon BJ, Dixon JB, Carden JR, Burn AJ, Schachter LM, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005; 102: 1110-1115. Ref.: https://tinyurl.com/y9a8hg7g
- Dhonneur G, Abdi W, Ndoko SK, Amathieu R, Risk N, et al. Video-assisted versus conventional tracheal intubation in morbidly obese patients. Obes Surg. 2009; 19: 1096-1101. Ref.: https://tinyurl.com/yd5smrz2
- Sundqvist J, Walldén J. PONV in bariatric surgery: 1AP5-2. Eur J Anaesthesiology. 2014; 31: 16-17. Ref.: http://bit.ly/2XHKnzQ
- Thorell A, Mac Cormick AD, Awad S, Reynolds N, Roulin D, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016; 40: 2065-2083. Ref.: https://tinyurl.com/y8d9bdzb
- Sinha A, Jayaraman L, Punhani D, Chowbey P. Enhanced Recovery after Bariatric Surgery in the Severely Obese, Morbidly Obese, Super-Morbidly Obese and Super-Super Morbidly Obese Using Evidence-Based Clinical Pathways: a Comparative Study. Obes Surg. 2017; 27: 560-568. Ref.: https://tinyurl.com/yc5p96m4